Synthesis of Imide-Acrylates and Their Applications for UV Curable Resins
Leave a CommentSynthesis of Imide-Acrylates and Their Applications for UV Curable Resins
Eiichi Okazaki, Tetsuji Jitsumatsu TOAGOSEI CO., LTD
1-1 Funami-Cho, Minato-Ku, Nagoya 455 Japan
Tel: 052-611-9910 Fax: 052-613-1868
I.ABSTRACT
Several kinds of imide-acrylates were synthesized and were measured their physical properties. They were applied for UV curable resins. It was found that these irnide-acrylates have excellent UV curability and their cured films have good flexibility and adhesion for many kinds of substances.
Moreover, it was found that the acrylate with a maleimide-group can be cured without conventional photoinitiators. It is interesting that the maleimide-acrylate would be able to improve durability, odor, toxic problems that are caused by photocleavage of photoinitiators after UV irradiation.
II.INTRODUCTION
UV curing technology has been utilized widely and esectively for numerous industrial applications, because UV cure system could bring us various advantages as follows, zero VOC, excellent productivity, superior physical properties, reduction of energy. Recently, this system has been accepted for not only coatings and inks of wood, metal and plastic but also technique of manufacturing LCD panel[1] and photofabrication of 3D model[2] Therefore, new materials which have an unique chemical or physical properties have been desired.
Today, most of the materials that have been applied to UV curable resins are monomers and oligomers including acrylic group for radical polymerization. Although some new cationic UV coating systems have introduced recently in order to improve the physical or chemical property, it isn’t sufficient for the newer various apphcations.
Acrylates including imide-group were focused because the group has strong chemical bond and high polarity for improving the properties. Then, several kinds of imide-acrylates were synthesized and were measured their physical properties as new unique UV curable monomers which would be able to be applied to newer various industrials.
In this report, acrylate with maleimide-group was attempted to apply photoinitiator- free system, because N-substituted maleimides are eificient photoinitiators of radical polymerization through the mechanism of hydrogen abstraction by excited N-substituted maleimide under UV irradiation.[3]
III.EXPERIMENTAL
I.SYNTHSIS OF IMIDE-ACRYLATES
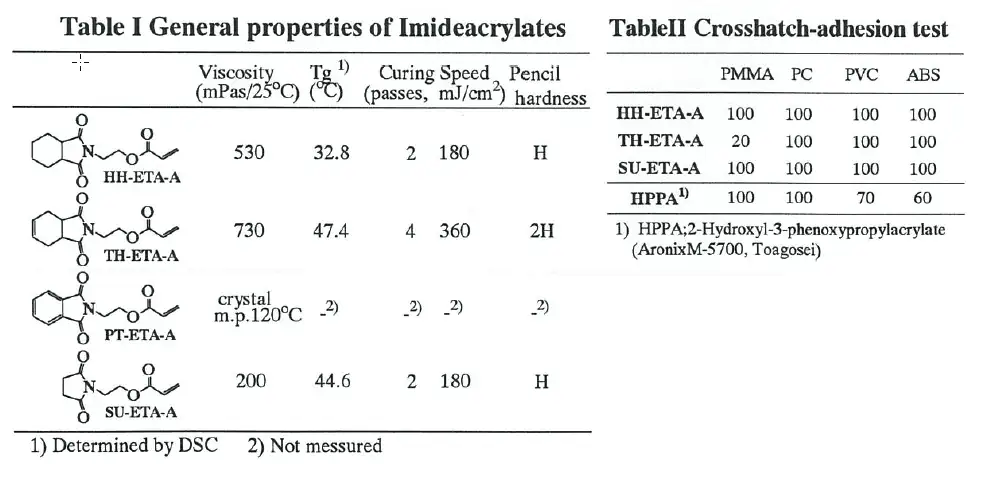
Imide-acrylates were synthesized from cyclic anhydride, amino alcohol and acryhc acid on the same way which Azuma had reported .[4] Synthesized compounds were shown in Tablet. PT-ETA-A which was synthesized from phthalic anhydride was crystal and difficult to dissolve in typical acrylates.
II.PROCEDURES
Tensile strength. elongation and Tg
Formulations of imide-acrylate added 1wt% of benzildimethylketa1(BDK) were poured into EPDM rubber mold to gain their films of lmm and were cured in air under 60W/cm° medium pressure mercury vapor lamp with an irradiation of 5,000mJ/cm°. Tensile strength and elongation were measured in accordance with Japanese Industrial Standard (JIS) K6301. Tg was measured by DSC.
Curine speed. pencil hardness and adhesion
Imide-acrylate formulations added 2wt% of BDK were drawn down on several kinds of plates using a 10-micron wire bar. The coatings were cured in air under 80W/cm2 medium pressure mercury vapor lamp at 90mJ/cm2 by 1 pass. Curing speed was indicated the number of passes which the tackiness of coating surface was completely disappeared on metal plate. Pencil hardness and crosshatch tape adhesion test were conducted in the same way of JIS KS400 on metal and some plastic plates, respectively.
IV. RESULTS AND DISCUSSION
I.PROPERTIES OF IMIDE-ACRYLATES
Table I gives the curing speed, pencil hardness and Tg of imide-acrylate itself. In spite of monofunctional acrylate, all imide-acrylates could be cured quickly and the essential dosage to cure were within 360mJ/cm°. The pencil hardness were relative hard and the level was from H to 2H.
The reason why imide-acrylate could be cured quickly was due to the reducible effect of inhibition of radical polymerization by oxygen in air, like a tertiary amine[5], because
Decker had reported that acrylate with a carbamate-group, similar heterocyclic compounds, could be cured excellently by the same reason.[6]
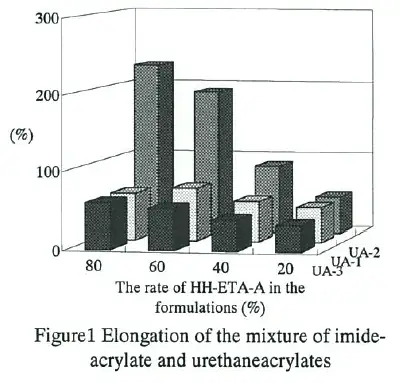
The result of adhesion test was shown in Table II. 2-Hydroxy-3-phenoxypropy1 acrylate(HPPA) was used for this test as a typical good adhesion monomer. Adhesion of imide-acrylates was better than HPPA for several kinds of plastics. This can be attributed to the significantly higher polarity that imide group has.
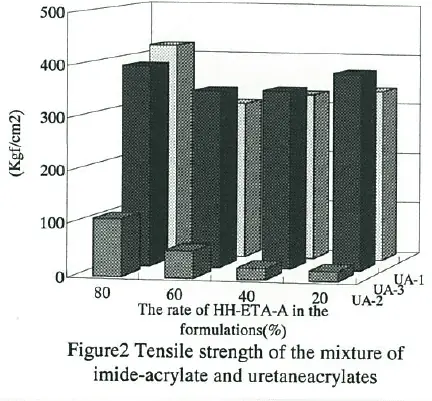
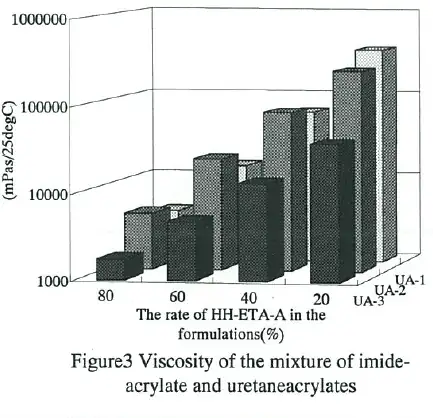
The tensile strength, elongation and viscosity of formulations which consist of HH- ETA-A and some conventional urethane acrylates are shown in Fig.1-3, respectively. UA- 1(Aronix M-1100, Toagosei) and UA-2(Aronix M-1310, Toagosei) are typical aromatic polyester urethaneacrylates. And UA-3(Aronix M-1600, Toagosei) is typical aliphatic polyether urethaneacrylate. Though HH-ETA-A was a monofunctional acrylate, both tensile strength and elongation were not spoiled, but partially improved by adding to urethaneacrylates.
This can be aho attributed to the higher polarity of imide group. It was reasonable that the viscosity was decreasing and then treating was easier by adding imide-acrylate to the formulations.
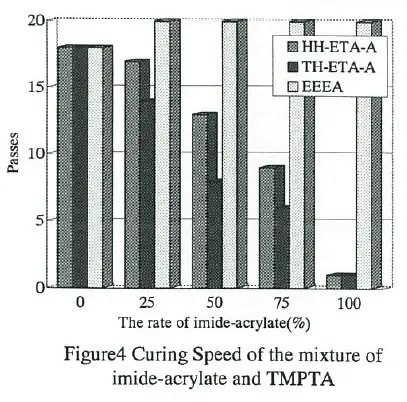
Fig.4 shows the comparison between imide-acrylates and ethoxyethoxyethyl acrylate(EEEA), typical monoacrylate, as a diluent. It was found that curing speed was improved excellently by adding imide-acrylate in the case of TMPTA.
II.APPLICATION FOR PHOTOINITIATOR -FREE SYSTEM
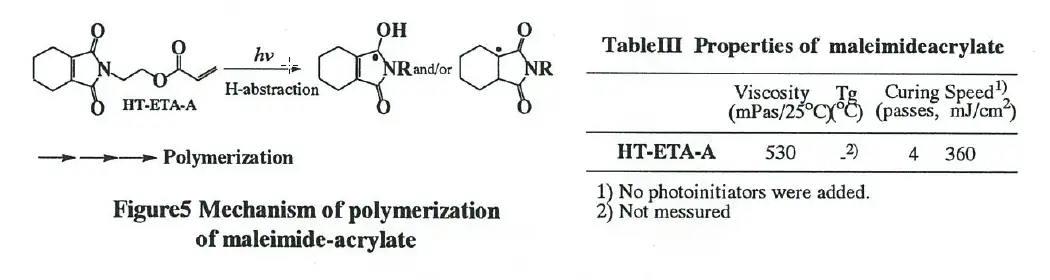
Recently, N-substituted maleimides are efficient photoinitiators of radical polymerization through the mechanism of hydrogen abstraction by excited N-substituted maleimide.[3] HT-ETA-A which consists of 2,3,4,5-tetrahydrophthalic anhydride, ethanolamine and acrylic acid has maleimide moiety, therefore it is expected that this imide- acrylate can polymerize itself and other acrylates without conventional photoinitiator.
Curing speed and pencil hardness of HT-ETA-A were shown in Table III. It was found that this acrylate can be cured fast enough, within 360mJ/cm°, without typical photoinitiator expectedly. Fig.5 shows the mechanism of polymerization through hydrogen abstraction by excited HT-ETA-A without photoinitiators under UV irradiation.
V.CONCLUSION
In this report, we have presented the results related to the properties of imide- acrylates as UV curable materials. We found that imide-acrylates have excellent UV curability, and their cured films have good 0exibiIity and adhesion for many kinds of substances.
Moreover, it was found that the acrylates which has a maleimide-group can be cured without conventional photoinitiators. It is interesting that these maleimide-acrylates would be able to improve durability, odor, toxic problems that are caused from photocleavage of conventional photoinitiators after UV irradiation.
VI.REFERENCES
- Fushimi T., Kobunshi, 45, 794, (1996) 2)
- Ikuta K., ibid, 45, 800, (1996)
- Jonsson S.E., Hultgren , Sundell P., Shimose M., Owens J., Vaughn K., Hoyle C.E.,
Radtech Europe95 academic day, 34, (1995)
Clark S. C., Jonsson S. E., Hoyle C. E., Polymer Preprints, 38(2), 363, (1997) - Azuma K., Kato , Tatemichi H., Kimura K., Japanese Laid-Open Patent Application (Kokai) No. 59481, (1975)
- Selli E., Bellobono I.R., Radiation Curing in Polymer Science and Technology vol.3 Polymerisation Mechanisms, Fouassier J.P. and Rabek J.F., Elsevier, London, UK,1993, p.18
- Decker C., ibid, p.33
Decker C., Elzaouk B., Decker C., Radtech Europe95 academic day, 115, (1996)