Development of Acrylates using New Ester Exchange Reaction
Kazuki Ofusa, Naoki Hashimoto
Toagosei Co., Ltd. 8, Showa-cho, Minato-ku, Nagoya, Japan
Abstract–A new method using an ester exchange reaction instead of the conventional dehydrative esterification reaction has been established to produce multifunctional acrylates. This method can provide lower viscosity and higher purity products compared to the conventional multifunctional acrylates.
Based on this method, new acrylates were synthesized from plant-derived raw materials, such as glycerol and sorbitol, and evaluated as light-curing resins. Glycerol triacrylate obtained via the direct acrylation of glycerol showed lower viscosity, higher hardness, and higher adhesion than the conventional acrylates with three or more functionalities. Ethylene oxide modified sorbitol
acrylate obtained from sorbitol as raw material is a multifunctional acrylate with high water solubility and has better properties than the conventional hydrophilic acrylates in terms of ultraviolet (UV) curability, coating film hardness, and water reducibility.
I. INTRODUCTION
Multifunctional acrylates widely used as light-curing resins are mainly synthesized via the dehydrative esterification method using alcohol and acrylic acid as raw materials and a strong acid (e.g.: sulfuric acid, p-toluenesulfonic acid) as a catalyst. However, the disadvantage of this method is that the product is likely to change over time due to catalyst residue and side reactions such as Michael addition can lead to high molecular weight and viscosity [l].
These problems can be solved by switching the method to an esterification process that does not use strong acids as a catalyst but the conventional esterification process has not been applied to the synthesis of 3 or higher functional acrylates due to problems such as low catalyst activity and difficulty in removing the catalyst [2].
The authors successfully synthesized acrylates with three or more functionalities, which were previously difficult to produce, using an independently developed ester exchange method. The acrylates produced by this method will be described below.
II. EXPERIMENT
2.1 Materials
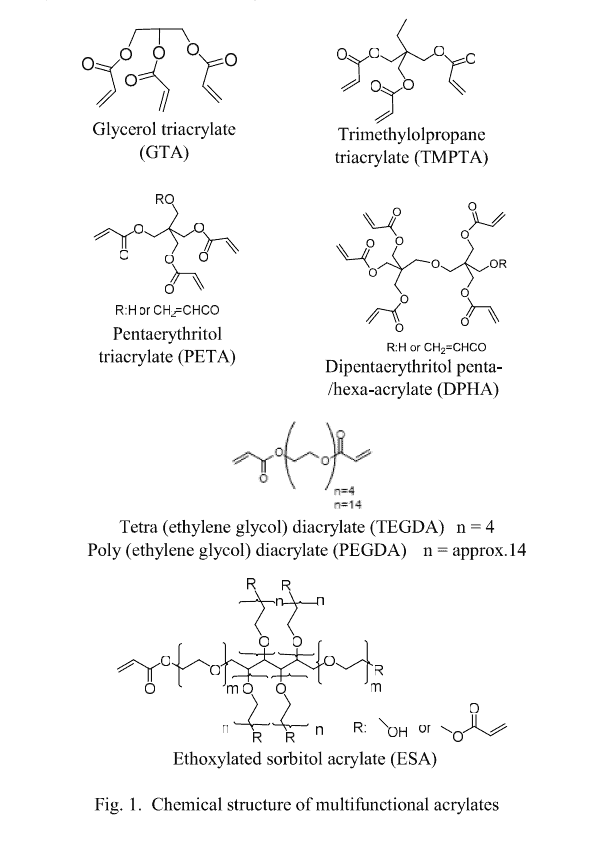
Dipentaerythritol penta-/hexa-acrylate (DPHA), glycerol triacrylate (GTA), and ethoxylated sorbitol acrylate (ESA) were synthesized via the ester exchange reaction with reference to the patents [3] [4]. This DPHA was designated as DPHA-1 to distinguish it from the conventional products.
For tetra (ethylene glycol) diacrylate (TEGDA), trimethylolpropane triacrylate (TMPT A), and pentarythritol triacrylate (PETA), “ARONIX M-240,” “ARONIX M-309,” and “ARONIX M-305,” respectively, manufactured via Toagosei were used without any modifications. For DPHA synthesized via the dehydrating esterification method, “ARONIX M-402” manufactured via Toagosei was used as it is, and it was designated as DPHA-2.
For poly(ethylene glycol) diacrylate (n approximately 14) (PEGDA), “Light Acrylate 14EG-A” manufactured by Kyoeisha Chemical was used as it was.
Figure 1 shows the structure of polyfunctional acrylates used in this study.
2.2 Evaluation of the Properties of Coated Films
A monomer/2-methyl-l-[4-(methylthio) phenyl]-2- morpholino-l-propanone (100 parts/5 parts) mixed solution was prepared and applied to an enhanced adherent PET film (Cosmoshine A-4300, made by
Toyobo, 50 μm) using a bar coater to obtain a film thickness of 10 μm. The film was then UV irradiated in air with a medium-pressure mercury vapor lamp (UV dose 800 mJ/cm2, maximum UV intensity 500 mW/cm2). Thereafter, the physical properties of the resulting coating films were evaluated by the following methods.
The pencil hardness was measured according to IlS K 5600-5-4.
Martens hardness was measured using a Fischer Scope H-lO0C ultra-micro hardness tester manufactured by Fischer Instruments, with a Vickers indenter, a maximum indentation load of 10 mN, and 10 s to reach the maximum load, and it was calculated from the maximum deformation of the coating film.
The scratch resistance was evaluated by rubbing the surface of the coating film with steel wool #0000 (under conditions of 1 kg load, reciprocating 100 times), and observing the appearance. If the appearance of the coating film after the test did not change at all, it was qualified as “excellent,” and if less than 10 scratches were observed, it was rated as “good.”
The adhesion was measured according to IlS K 5600- 5-6 (cross-cut test). Toyobo “Cosmoshine A-4300” (50 μm) (as enhanced adhesion treated polyethylene
terephthalate (PET)), Teijin “Panlite PC-2151” (125 μm) (as polycarbonate (PC)), and “FUilTAC-80UL” (80 μm) manufactured via FUilFILM Corporation (as triacetylcellulose (TAC)) were used as they were.
2.3 UV-LED Curability
An acrylate/diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (100 parts/10 parts) mixed solution was prepared and applied to an art paper sheet (manufactured via Mitsubishi Paper Mills) using a bar coater to set a film thickness of 5 μm. UV irradiation was then performed with a UV-LED (385 nm) (maximum UV intensity 200 mW/cm2). The UV dose was adjusted by varying the conveyor speed, the coating film surface was rubbed with a nonwoven cloth, and the UV-LED curability was defined as the lowest UV dose in which no scratches were observed on the film.
Ill. RESULTS AND DISCUSSION
3.1 DPHA
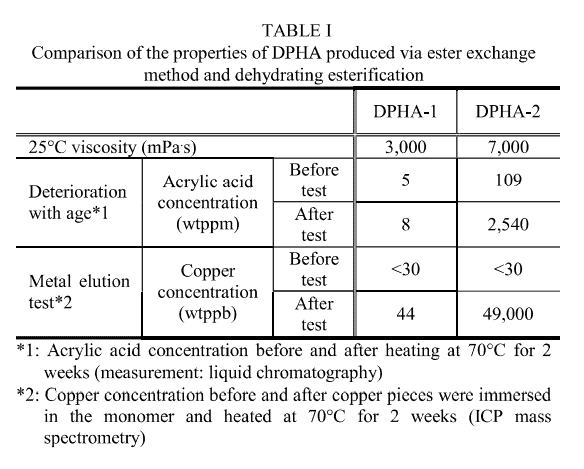
Dipentaerythritol penta-/hexa-acrylate (DPHA) is extensively used in inks, coatings, and solder resists owing to its outstanding hardness and curability among multifunctional acrylates. However, conventional DPHA is produced via the dehydrative esterification method, which causes problems, including high molecular weight and viscosity, due to side reactions such as Michael addition as well as change in quality over time and metal corrosion because of the presence of a residual strong acid catalyst [ 1].
A comparison of the physical properties of DPHA-1 synthesized by the ester exchange method and DPHA-2 produced by the dehydrating esterification method is shown in Table I. DPHA-1 exhibits much lower viscosity due to a reduced amount of high molecular weight products formed via Michael addition, and deterioration with age and metal corrosion resistance are improved due to the absence of a residual strong acid catalyst. These characteristics make DPHA synthesized by the ester exchange method promising for automotive equipment and resist applications, which require high moisture/heat resistance and insulation properties.
3.2 GTA
Glycerol is a trivalent alcohol obtained from biomass animal fats or vegetable oils, and glycerol triacrylate (GTA), which is produced by acrylation of glycerol, is preferred from a carbon-neutral perspective. However, as glycerol has a hardly esterifiable secondary hydroxyl group, it has been difficult to synthesize high-purity GT A with conventional methods.
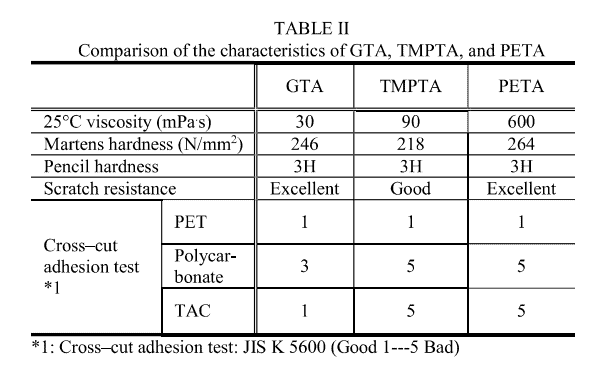
The authors succeeded in synthesizing high-purity GTA using the abovementioned ester exchange method. Table II compares the physical properties of the obtained GTA with those of trimethylolpropane triacrylate (TMPTA) and pentarythritol triacrylate (PETA) produced via conventional methods.
GT A showed substantially lower viscosity than TMPTA and PETA This can be explained by the smaller molecular weight of the ideal structure in GT A compared to that of TMPT A and PETA and by the suppression of increase in molecular weight caused by Michael addition. Upon comparing the physical properties of the cured products, GTA proved superior to TMPTA in both Martens hardness and scratch resistance, and its performance was similar to that of PETA, which contains tetra:functional acrylate with a higher crosslinking density.
Furthermore, the results of the cross-cut adhesion test showed that GTA has better adhesion than TMPTA and PET A. It has been reported that penetration into the base material is an important factor in the adhesion of light- curing resins to plastics [5], and it is assumed that GTA shows better penetration ability into plastics than TMPTA and PETA because of its low viscosity and molecular weight.
Summarizing the above results, GT A is a multifunctional acrylate characterized by lower viscosity, higher hardness, and higher adhesion compared to the conventional multifunctional acrylates and it can be considered superior in terms of carbon neutrality.
3.3 ESA
Sorbitol is a biomass-derived polyhydric sugar alcohol obtained by the reduction of glucose produced by starch hydrolysis. It has been reported that acrylate of 6 moles EO adduct of sorbitol, in which an average of 1 mole of ethylene oxide (EO) is added to the hydroxyl groups of sorbitol, has excellent UV curability [6]. Recently, considering the reduction of environmental load, an increasing need emerged for UV-curable resins that use water as a diluent in paints to diminish VOCs. As sorbitol is a hexafunctional alcohol, by reducing the acrylation rate and leaving hydroxyl groups, it becomes possible to obtain multifunctional acrylates with high water solubility. However, because the dehydrative esterification method requires a water washing process to remove the residual acrylic acid and strong acid catalyst, it is difficult to retain the water-soluble components in the product.
The authors synthesized highly water-soluble ethoxylated sorbitol acrylate (ESA) by the ester exchange method that does not require the abovementioned water washing process, and evaluated its physical properties.
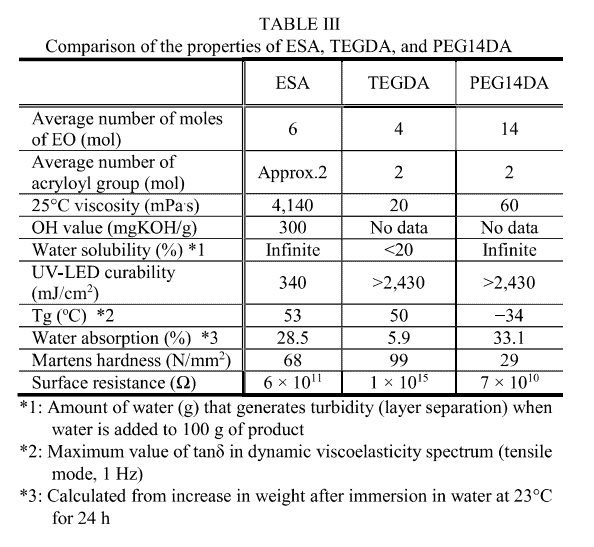
Because ESA synthesized this time has a comparatively small average number of 2 acryloyl groups per molecule and an extremely high hydroxyl value of 300 mg KOH/g, it can be dissolved in water at any ratio.
Table III shows the results of comparison of ESA with polyethylene glycol (n = 14) diacrylate (PEG14DA), a commercially available water-soluble multifunctional acrylate, and tetraethylene glycol diacrylate (TEGDA), a hydrophilic bifunctional acrylate with a relatively high Tg.
When comparing UV curability, ESA proved much better than PEG14DA and TEGDA. The plausible reason is that ESA is relatively less affected by oxygen inhibition due to its hydroxyl groups [7].
The Tg of the ESA cured product was much higher than that of PEG14DA and comparable to that of TEGDA, and the Martens hardness of ESA was also
higher than that of PEG14DA.
Comparing the water absorption of the cured films, the water absorption of ESA was about 30 wt%, which is equivalent to that of PEG14DA, and the hydrophilicity
was very high. When measuring the surface resistance of the cured coating film, it was much lower for ESA than for TEGDA and close to PEG14DA. The probable reason is that since ESA and PEG14DA give highly hydrophilic cured products, moisture is easily adsorbed on their coating film surfaces.
These results suggest that ESA has better properties than the conventional hydrophilic acrylates in terms of UV curability, coating film hardness, and water solubility, and it is useful as a raw material for water-soluble UV paints and antistatic UV coatings as well as antifog coatings that require hydrophilic properties.
IV. CONCLUSIONS
This paper succeeds in synthesizing acrylates with three or more functionalities, which have been difficult to obtain in the past, using the author-developed ester exchange method. The multifunctional acrylates produced via this method exhibit outstanding properties including lower viscosity, higher purity, and lower deterioration with age than those of the conventional products.
New acrylates obtained from plant-derived raw materials, glycerol, and sorbitol were synthesized via this process and evaluated as light-curing resins. The glycerol triacrylate showed lower viscosity, higher hardness, and higher adhesiveness than the conventional trifunctional acrylates. Furthermore, the EO modified sorbitol acrylate obtained from sorbitol is a multifunctional acrylate with high water solubility, which offers better properties than the conventional hydrophilic acrylates in terms of UV curability, coating film hardness, and water reducibility.